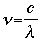Acids
In everyday life we deal with many compounds that chemists classify as acids. For example, orange juice and grapefruit juice contain citric acid. These juices, and others, also contain ascorbic acid, a substance more commonly known as Vitamin C. Salads are often flavored with vinegar, which contains dilute acetic acid. Boric acid is a substance that is sometimes used to wash the eyes.
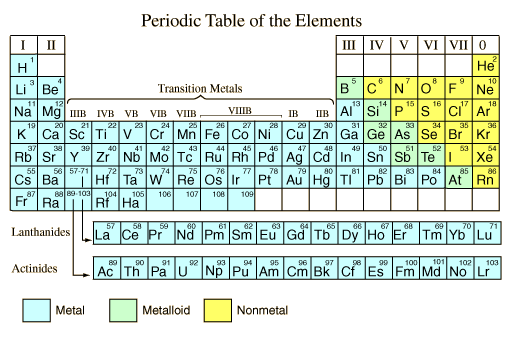
In any chemistry laboratory, we find acids such as hydrochloric acid, sulfuric acid, and nitric acid. These acids are called mineral acids because they can be prepared from naturally occurring compounds called minerals. Mineral acids are generally stronger than household acids, and should be handled with great care because they can burn skin and clothing.
Properties of Acids:
Acids taste sour. Citric acid is responsible for the sour taste of lemons, limes, grapefruits, and oranges. Acetic acid is responsible for the sour taste of vinegar.
Acids turn litmus (or indicator papers) red. Litmus is a vegetable dye that may be either red or blue, depending on the acidity. When a sample of an acid is placed on red litmus paper, the color of the litmus does not change. Red litmus has been previously treated with acid. Adding more acid does not change the red color. However, when the same acid is placed on blue litmus paper, the color turns from blue to red. (Blue litmus has been treated with a base).
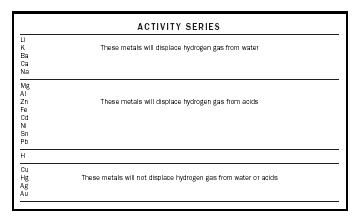
Acids contain combined hydrogen. When a sample of zinc, a fairly reactive metal, is dropped into a test tube containing an acid such as hydrochloric acid, a reaction occurs. The bubbling in the tube indicates that a gas is released. When we test this gas by inserting a burning splint into the test tube, the gas burst into flame and produces a small popping sound. This is the characteristic test for hydrogen gas. In general, when certain acids react with metals, hydrogen gas is released. See following reactions:
Zn (s) + 2HCl (aq) H2 (g) + ZnCl2 (aq)
Zn (s) + H2SO4 (aq) H2 (g) + ZnSO4 (aq)
Acids release hydrogen in water solutions. When an acid dissolves in water, the acid ionizes, releasing both hydrogen ions and ions of a nonmetal or nonmetallic polyatomic ion. Thus, when hydrochloric acid is dissolved in water, the acid ionizes, forming hydrogen ions and chloride ions, as shown in the following equation:
HCl (aq) H+ (aq) + Cl1- (aq)
Other examples:
H2SO4 (aq) 2 H+ (aq) + SO42- (aq)
H3PO4 (aq) 3 H+ (aq) + PO43- (aq)
Special example:
HC2H3O2 (aq) -> H+ (aq) + C2H3O21- (aq)
Note the use of the double arrow in the ionization of acetic acid. We know that acetic acid is a weak acid. The smaller arrow pointing to the right indicates that the change to the right (ionization) is relatively small. This means that, in a solution of acetic acid, we have a large number of acetic acid molecules and few hydrogens and few acetate ions.
Thus acids are defined as substances that release hydrogen ions in solution. It is these H+ (aq) that are responsible for the properties of acids.
How Acids are Prepared:
Acids may be prepared when certain gases, related to acids, are dissolved in water and when certain salt compounds, again related to acids, are allowed to react with sulfuric acid.
CO2 (g) + H2O (l) H2CO3 (aq) carbonic acid
2 NaCl (s) + H2SO4 (aq) + heat 2 HCl (g) + Na2SO4 (aq)
Uses of Acids:
Sulfuric acid is the chemical most widely used in industry. Sulfuric acid is also used to make other acids such as hydrochloric and nitric acid because the boiling point of sulfuric acid is higher that that of other acids. This allows the acid being produced to be distilled and collected separately from the starting material.
Sulfuric acid is also used to remove the surface oxide layers on metals (pickling) before the metals are coated with materials that prevent rusting. For example, before iron is coated with chromium (in chromium plating), the iron is dipped into dilute sulfuric acid to remove the iron oxide normally present on the surface of the iron. Another important use of sulfuric acid is the storage cell. In a lead storage cell, dilute sulfuric acid serves as the electrolyte through which ions move between the lead plates, acting as
the cathode, and the spongy lead dioxide, acting as the anode. Several such cells connected together make up the type of storage battery used in automobiles.
Nitric acid, another important industrial acid, is used in the manufacture of fertilizers, plastics, photographic film, and dyes. Nitric acid is also used in the preparation of such explosives as dynamite and TNT.
Hydrochloric acid, like sulfuric acid, is used to clean metals. Hydrochloric acid is also used to clean brick and tile; it is used in the manufacture of sugar and glue. Hydrochloric acid is produced in small quantities in the stomach where the acid aids digestion.
Bases:
Ammonium hydroxide, or ammonia water, is very irritating to the nose and the eyes. This substance, called a hydroxide, or a base, is often used in the home for cleaning because bases generally dissolve grease. Milk of magnesia (magnesium hydroxide), which is used as an antacid, is a base; lye (sodium hydroxide), which is used in the manufacture of soap, is another familiar example of base.
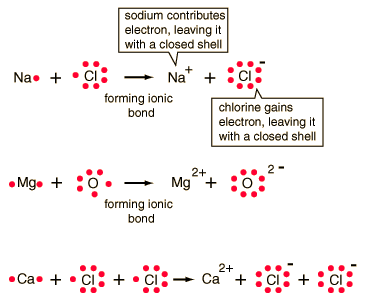
Bases are ionic compounds containing metal ions and hydroxide ions. For example, sodium hydroxide contains sodium ions and hydroxide ions. When sodium metal is placed in water, sodium hydroxide is formed and hydrogen gas is released. Since the formula for water can be written as HOH instead of H2O, the reaction involves single replacement:
2 Na (s) + 2 HOH (l) 2 NaOH (aq) + H2 (g)
Properties of Bases: (in water solutions)
1. Bases taste bitter. A bitter taste is characteristic of all bases. It is the presence of a base that give unflavored milk of magnesia its bitter taste.
2. Bases feel slippery. If you rub a drop or two of household ammonia between your fingers, you experience the slippery feeling of a base. Wet soap is also slippery because of the presence of a base.
3. Bases turn red litmus blue. A common indicator, used to detect the presence of a base, is phenolphthalein which, when mixed with a base, turns pink.
4. Bases release hydroxide ions in water solutions. When dissolved in water, bases ionize releasing metal ions (or metallic polyatomic ions) and hydroxide ions. For example: when sodium hydroxide is dissolved in water, it ionizes as:
NaOH (s) + H2O (l) Na1+ (aq) + OH1- (aq)
NH4OH (aq) -> NH41+ (aq) + OH1- (aq) double arrow indicates ionization of weak base ammonium hydroxide
Thus bases are defined as substances that release hydroxide ions in solution. It is these OH1- (aq) ions that are responsible for the properties of bases.
How Bases are Prepared:
Bases may be prepared by the reaction of water and very reactive metals, related to the base, and by the reaction of water and certain oxides, again related to the base.
2 K (s) + 2 HOH (l) 2 KOH (aq) + H2 (g)
CaO (s) + HOH (l) Ca(OH)2 (aq) plus considerable heat released
Uses of Bases:
Ammonium hydroxide, frequently called ammonia, is used in the preparation of important related compounds such as nitric acid and ammonium chloride. Ammonia is also used as a cleaning agent.
Sodium hydroxide is used in the manufacture of soap, rayon, and paper. Strong solutions of this base are very caustic; that is, they are extremely harmful to the skin.
Calcium hydroxide, commonly known as slaked lime, is used in the preparation of plaster and mortar. Water solutions of calcium hydroxide, called limewater, can be used in the lab as a test for the presence of carbon dioxide.
Salts:
Many chemical compounds may be classified as salts. The salt most familiar to all of us is table salt -- sodium chloride. Baking soda is the salt sodium bicarbonate. Magnesium sulfate, also called Epsom salts, is often found in the home.
In general, salts are ionic compounds that are composed of metallic ions and nonmetallic ions. For example, sodium chloride is composed of metallic sodium ions and nonmetallic chloride ions. Some salts are composed of metallic polyatomic ions and nonmetallic polyatomic ions (ammonium nitrate is composed of ammonium ions and nitrate ions).
Properties of Salts:
The salty taste of ocean water is due to the presence of salts such as sodium chloride and magnesium bromide. There are many different salts present in salt water:
salt in saltwater
formula
percentage in saltwater
sodium chloride
NaCl
(2.72 %)
magnesium chloride
MgCl2
(0.38%)
magnesium sulfate
MgSO4
(0.17 %)
calcium sulfate
CaSO4
(0.13 %)
potassium chloride
KCl
(0.09 %)
calcium carbonate
CaCO3
(0.01 %)
magnesium bromide
MgBr2
(0.01 %)
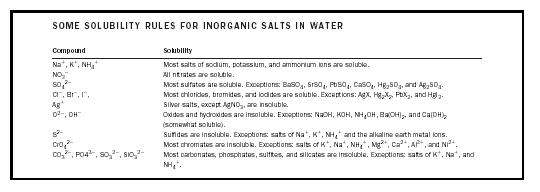
Salts dissociate in water. Salts consist of tightly bonded ions. In water, these bonds are weakened and the ions become mobile. This accounts for the fact that salt solutions are generally electrolytes. In water, for example, sodium chloride ionizes, or dissociates like this:
NaCl (s) Na1+ (aq) + Cl1- (aq)
Salts may react with water. Some salt solutions, when tested with litmus show acid, others base, and others still nothing. How does this happen?
1) When sodium carbonate dissolves in water, the salt liberates sodium ions and carbonate ions. At the same time, the water itself ionizes slightly to form hydrogen and hydroxide ions (remember that water is a weak electrolyte):
Na2CO3 (s) 2 Na1+ (aq) + CO32- (aq) HOH H1+ (aq) + OH1- (aq)
2) Thus, the following particles may be present in a solution of sodium carbonate: water molecules, sodium ions, carbonate ions, hydrogen ions, and hydroxide ions. The ions of opposite charge attract one another and combine to form sodium hydroxide and carbonic acid:
2 Na1+ (aq) + CO32- (aq) + 2 H1+ (aq) + 2 OH1- (aq) 2 NaOH (aq) + H2CO3 (aq)
The reaction of a salt and water to form an acid and a base is called a hydrolysis reaction. Since acids and bases react to form water and salt (neutralization reactions) hydrolysis reactions are the reverse of neutralization reactions. Thus, when sodium carbonate is dissolved in water, carbonic acid and sodium hydroxide are formed. Carbonic acid, H2CO3 is the acid present in soda water. Since carbonic acid decomposes on standing to form CO2 gas and H2O, it is called a weak acid. From conductivity experiments we know that sodium hydroxide, NaOH, is a strong base.
Na2CO3 (aq) + 2 HOH (l) H2CO3 (aq) + 2 NaOH (aq)
When the acid formed in a hydrolysis reaction is stronger that the base, the effect of such a solution on litmus is that of an acid. For example; a water solution of the weak base, ammonium hydroxide and water, produces ammonium hydroxide (a weak base) and hydrochloric acid ( strong acid). Ammonium hydroxide, on standing, decomposes to form gaseous ammonia, NH3.
When both the acid and the base in a hydrolysis reaction are equally strong (or equally weak), the effect of such a solution on litmus is neither that of an acid or a base. For example, when sodium chloride is dissolved in water and the solution is tested with litmus, neither color changes, indicating that the solution is neither acidic nor basic. Such a solution is said to be neutral and the sodium chloride has not undergone hydrolysis.
Uses of Salts:
Name of salt
Formula
Uses
ammonium chloride
NH4Cl
in soldering, as electrolyte in dry cells
sodium bicarbonate
NaHCO3
in baking powder, in manufacture of glass
sodium chloride
NaCl
for seasoning and preserving food, essential in life processes
calcium chloride
CaCl2
as a drying agent to absorb moisture, in freezing mixtures
silver bromide
AgBr
in making photographic film
potassium nitrate
KNO3
in manufacture of explosives; fertilizer
sodium nitrate
NaNO3
fertilizer; source of nitric acid
Preparation of Salts:
Salts may be prepared by three methods:
1) neutralization of acid and base -- When an acid and base react, they counteract each other, that is, they neutralize each other. Such a reaction, known as a neutralization reaction, results in the formation of water and a salt. For example, when sodium hydroxide and hydrochloric acid react, water and the salt sodium chloride are formed. This occurs because the hydrochloric acid and the sodium hydroxide first ionize, and then react. The compounds ionize releasing hydrogen, chloride, sodium, and hydroxide ions.
Since these are mobile in solution, hydrogen ions meet hydroxide ions and unite to form water. At the same time sodium ions and chloride ions remain as aqueous salt.
2) Direct combination -- When a metal reacts with a nonmetal, a salt is generally formed. For example, when the metal magnesium is burned in chlorine gas, the salt magnesium chloride is formed.
3) Metal oxide and acid -- when a metal oxide reacts with an acid, a salt is formed. For example, when calcium oxide reacts with nitric acid, the salt calcium nitrate is formed.
Acids, Bases, Salts, pH, Buffers, Indicators, Titration, Antacids
Acids Acids are substances which ionize in water solutions to produce hydrogen ion (H+ or free p+). Some ionize more than others. Acids taste sour and turn litmus red.
Bases Bases are substances which ionize to produce hydroxide ions in water solutions. They taste bitter, feel slippery to the touch, and turn litmus blue.
Salts Salts are crystalline compounds composed of the negative ions of an acid and the positive ions of a base.
Hydronium ion H+ combines with a water molecule to form H30+ any solution containing hydronium ions is acidic and the strength of an acid is based on the number of hydronium ions in solution.
Ionization Ionization is the formation of ions in solution.
Dissociation Dissociation is the separation of ions in solution.
Neutralization Neutralization is the reaction of an acid and a base to form a salt plus water.
Hydrolysis Hydrolysis is a reaction of a salt with water to form a weak acid or base.
Titration Titration is a technique for measuring the relative strength of a solution.
Endpoint Endpoint is the point in a titration where equal amounts of reactants are present.
Buffer A buffer is a solution which can receive moderate amounts of of either acid or base without significant changes in pH.
Indicator An indicator is used to indicate the pH of a solution.
pH or pOH pH is the negative log of the hydrogen ion concentration. pOH is the negative log of the hydroxide ion concentration.
A weak acid/base ionizes only slightly in solution whereas a strong acid/base completely ionizes (dissociates) into positive and negative ions in solution.
Demonstration Procedure:
I. heat 75 mL of 0.1 M HCI on hot plate
II. crush antacid tablet and add this to acid
III. add solution to graduated cylinder with enough water to have 100 mL
IV. place 25 mL of solution in Erlenmeyer flask
V. add phenolphthalein as indicator
VI. titrate with 0.1 M NaOH to endpoint
VII. test solution with calibrated pH meter (using buffer solutions)
Hydrogen Ions, pH, and Indicators
Background:
The hydrogen ion concentration [H+] in water solutions can vary over a wide range of values. These values may be over 1 Molar [H+] or less than 1 E – 14 Molar [H+]. To deal with such large possible variations a mathematical conversion of H+ to a logarithmic value has been used to express the H+ concentration as a pH.
pH = -Log10[H+]
As you know perhaps, a logarithm is the exponent of a base number which makes the exponential form equal to another natural number. For example [102 = 100], if the base number 10 has an exponent of 2, the expression is equal to 100. The exponent 2 is the logarithm of 100. The logarithm of 1000 would be 3 [103 = 1000]. In the base 10 system, a difference of one in two logarithmic values would mean a 10 fold difference in the values of the two natural numbers which they represent, i.e., the logarithms of 2 and 3 represent 100 and 1000. There are logarithm tables which will give decimal logarithmic values so that any natural number can be represented. [101.699 = 50; the logarithm of 50 is 1.699].
The variation of the value of the [H+] of 1 to 10-14 molar can be expressed as pH values of 0 to 14. Neutral water has a [H+] of 1 E –7 M and therefore has a pH of 7. Acidic solutions will have pH values less than 7 and basic solutions will have pH values greater than 7.
The pH of a solution can be used to describe the acidic or basic qualities of soil samples, cosmetics, food, beverages, etc.
The pH can be determined by electrical devices which have electrodes which are sensitive to the hydrogen ion concentration (pH meter) or by certain compounds which change color over certain pH ranges.
Indicators are weak organic acids or bases which change color (and structure) with a change in pH.
pH and Indicators
The concentration of hydrogen ion is a measure of the acidity and the basicity of a solution. The concentration of hydrogen ion may be expressed in terms of the molarity of the acid or base solution; however, it is frequently more convenient to express the concentration as a function of the hydrogen ion concentration, pH. The pH of a solution may be defined as the exponent of the hydrogen ion concentration. This definition may be stated mathematically as:
pH = - log [H+] where [H+] is the molar hydrogen ion concentration.
The pH scale for water systems ranges from a value to 0 to 14 only. See the table that follows for the strength of the acid or base in water at 25º C.
pH range
[H+] range
Strength
0 to 2
1 E 0 to 1 E - 2
Strong acid
3 to 4
1 E – 3 to 1 E – 4
Moderate acid
5 to 6
1 E – 5 to 1 E – 6
Weak acid
7
1 E – 7
Neutral
8 to 9
1 E – 8 to 1 E – 9
Weak base
10 to 11
1 E – 10 to 1 E – 11
Moderate base
12 to 14
1 E – 12 to 1 E - 14
Strong base
Indicators have been developed in order to assist in the determination of the pH of a solution. These indicators are weak organic acids or bases which have the property of changing color in solution when the hydrogen ion concentration reaches a definite value. An acid indicator may be represented by the equation: HIn = H+ + In-
The anion, In-, represents a complex organic group which has changed its structure due to the loss of a hydrogen ion. The loss of the hydrogen ion is accompanied by a change in color. Since an indicator reaction is an equilibrium reaction, the addition of hydrogen ions would force the above reaction to the left and a color indicating an acid solution would result. The addition of hydroxide ions would cause the reaction to go to the right and a color associated to a basic solution would result.
The pH ranges of some indicators are given in the following table. With this table you can estimate the pH of a solution. Suppose phenolphthalein in introduced into a solution and the color of the solution becomes red. This red color indicates that the pH of the solution is 10.0 or higher. If indigo carmine is added to a new sample of the same solution and a blue color results, the pH will be narrowed to a range of 10.0 to 11.4, since the lower limit of color change for indigo carmine is blue. By using an additional indicator or indicators and a new sample of the solution, the pH of the solution can be narrowed to a small range.
Indicator
pH Range
Color Range
Methyl violet
0.0 – 1.6
Yellow to blue
Thymol blue
1.2 – 2.8
Red to yellow
Methyl orange
3.2 – 4.4
Red to yellow
Congo red
3.0 – 5.0
Blue to red
Methyl red
4.8 – 6.0
Red to yellow
Phenol red
6.6 – 8.0
Red to blue
Litmus
4.7 – 8.2
Red to blue
Cresol red
7.4 – 8.6
Yellow to red
Phenolphthalein
8. 2 – 10.0
Colorless to red
Thymolphthalein
9.4 – 10.6
Colorless to blue
Alizarin yellow R
10.1 – 12.0
Yellow to red
Indigo carmine
11.4 – 14.0
Blue to yellow


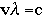
 = wavelength in units of nanometers (nm)
= wavelength in units of nanometers (nm)